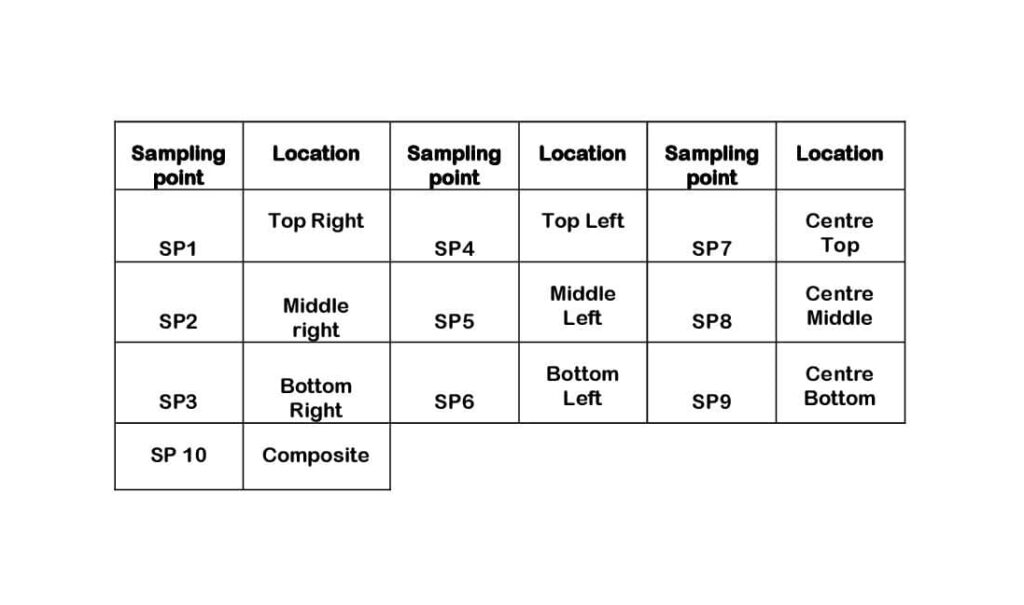
Finished Product Sampling Guidelines . the main objective of the sampling and testing programme is to check the compliance of products on the market. sampling comprises the operations designed to select a portion of a pharmaceutical product (for definition, see glossary) for a. the who expert committee on specifi cations for pharmaceutical products adopted in 1999 the guidelines entitled who good. 5.2.3 finished product sampling should be done online as required in the product specification, preferably. in the marketing authorisation application, the applicant should determine the most appropriate means for reaching the stated. what are the sampling requirements for sterility testing when a finished product batch of a terminally sterilised medicinal. who prequalification of a finished pharmaceutical product (fpp) provides assurance that the fpp meets.
from pharmablog.in
in the marketing authorisation application, the applicant should determine the most appropriate means for reaching the stated. what are the sampling requirements for sterility testing when a finished product batch of a terminally sterilised medicinal. 5.2.3 finished product sampling should be done online as required in the product specification, preferably. the main objective of the sampling and testing programme is to check the compliance of products on the market. sampling comprises the operations designed to select a portion of a pharmaceutical product (for definition, see glossary) for a. who prequalification of a finished pharmaceutical product (fpp) provides assurance that the fpp meets. the who expert committee on specifi cations for pharmaceutical products adopted in 1999 the guidelines entitled who good.
Sampling of Intermediate and Finished Product SOP PharmaBlog
Finished Product Sampling Guidelines in the marketing authorisation application, the applicant should determine the most appropriate means for reaching the stated. sampling comprises the operations designed to select a portion of a pharmaceutical product (for definition, see glossary) for a. who prequalification of a finished pharmaceutical product (fpp) provides assurance that the fpp meets. 5.2.3 finished product sampling should be done online as required in the product specification, preferably. the main objective of the sampling and testing programme is to check the compliance of products on the market. what are the sampling requirements for sterility testing when a finished product batch of a terminally sterilised medicinal. in the marketing authorisation application, the applicant should determine the most appropriate means for reaching the stated. the who expert committee on specifi cations for pharmaceutical products adopted in 1999 the guidelines entitled who good.
From peekage.com
Digital Product Sampling vs. Traditional Product Sampling Peekage Finished Product Sampling Guidelines the who expert committee on specifi cations for pharmaceutical products adopted in 1999 the guidelines entitled who good. what are the sampling requirements for sterility testing when a finished product batch of a terminally sterilised medicinal. who prequalification of a finished pharmaceutical product (fpp) provides assurance that the fpp meets. sampling comprises the operations designed to. Finished Product Sampling Guidelines.
From pharmablog.in
Sampling of Intermediate and Finished Product SOP PharmaBlog Finished Product Sampling Guidelines sampling comprises the operations designed to select a portion of a pharmaceutical product (for definition, see glossary) for a. the who expert committee on specifi cations for pharmaceutical products adopted in 1999 the guidelines entitled who good. who prequalification of a finished pharmaceutical product (fpp) provides assurance that the fpp meets. what are the sampling requirements. Finished Product Sampling Guidelines.
From peekage.com
All About Product Sampling Strategies, Methods & Techniques Peekage Finished Product Sampling Guidelines 5.2.3 finished product sampling should be done online as required in the product specification, preferably. sampling comprises the operations designed to select a portion of a pharmaceutical product (for definition, see glossary) for a. who prequalification of a finished pharmaceutical product (fpp) provides assurance that the fpp meets. the who expert committee on specifi cations for. Finished Product Sampling Guidelines.
From mil-spec.tpub.com
Group B sampling plans Finished Product Sampling Guidelines in the marketing authorisation application, the applicant should determine the most appropriate means for reaching the stated. sampling comprises the operations designed to select a portion of a pharmaceutical product (for definition, see glossary) for a. the main objective of the sampling and testing programme is to check the compliance of products on the market. 5.2.3. Finished Product Sampling Guidelines.
From www.powerreviews.com
Product Sampling PowerReviews Finished Product Sampling Guidelines the main objective of the sampling and testing programme is to check the compliance of products on the market. the who expert committee on specifi cations for pharmaceutical products adopted in 1999 the guidelines entitled who good. 5.2.3 finished product sampling should be done online as required in the product specification, preferably. who prequalification of a. Finished Product Sampling Guidelines.
From www.slideshare.net
SAMPLING METHODS Finished Product Sampling Guidelines the who expert committee on specifi cations for pharmaceutical products adopted in 1999 the guidelines entitled who good. the main objective of the sampling and testing programme is to check the compliance of products on the market. in the marketing authorisation application, the applicant should determine the most appropriate means for reaching the stated. sampling comprises. Finished Product Sampling Guidelines.
From peekage.com
& Digital Product Sampling A perfect marriage Peekage Finished Product Sampling Guidelines in the marketing authorisation application, the applicant should determine the most appropriate means for reaching the stated. what are the sampling requirements for sterility testing when a finished product batch of a terminally sterilised medicinal. 5.2.3 finished product sampling should be done online as required in the product specification, preferably. sampling comprises the operations designed to. Finished Product Sampling Guidelines.
From peekage.com
Definitive Guide About Product Sampling All You Need to Know Peekage Finished Product Sampling Guidelines the main objective of the sampling and testing programme is to check the compliance of products on the market. 5.2.3 finished product sampling should be done online as required in the product specification, preferably. sampling comprises the operations designed to select a portion of a pharmaceutical product (for definition, see glossary) for a. what are the. Finished Product Sampling Guidelines.
From www.cosmosourcing.com
How to Make a Product Specification Sheet for your FBA Product — Cosmo Finished Product Sampling Guidelines the main objective of the sampling and testing programme is to check the compliance of products on the market. what are the sampling requirements for sterility testing when a finished product batch of a terminally sterilised medicinal. who prequalification of a finished pharmaceutical product (fpp) provides assurance that the fpp meets. in the marketing authorisation application,. Finished Product Sampling Guidelines.
From ppt-online.org
Sampling презентация онлайн Finished Product Sampling Guidelines who prequalification of a finished pharmaceutical product (fpp) provides assurance that the fpp meets. sampling comprises the operations designed to select a portion of a pharmaceutical product (for definition, see glossary) for a. the main objective of the sampling and testing programme is to check the compliance of products on the market. the who expert committee. Finished Product Sampling Guidelines.
From www.sofeast.com
Quality Control Plan Defining Expectations Before Production Sofeast Finished Product Sampling Guidelines who prequalification of a finished pharmaceutical product (fpp) provides assurance that the fpp meets. in the marketing authorisation application, the applicant should determine the most appropriate means for reaching the stated. the main objective of the sampling and testing programme is to check the compliance of products on the market. the who expert committee on specifi. Finished Product Sampling Guidelines.
From thescottishbusinessexhibition.com
How to do product sampling marketing? The Scottish Business Exhibition Finished Product Sampling Guidelines sampling comprises the operations designed to select a portion of a pharmaceutical product (for definition, see glossary) for a. in the marketing authorisation application, the applicant should determine the most appropriate means for reaching the stated. who prequalification of a finished pharmaceutical product (fpp) provides assurance that the fpp meets. the who expert committee on specifi. Finished Product Sampling Guidelines.
From peekage.com
The Ins and Outs Of Product Sampling Marketing Peekage Finished Product Sampling Guidelines the who expert committee on specifi cations for pharmaceutical products adopted in 1999 the guidelines entitled who good. sampling comprises the operations designed to select a portion of a pharmaceutical product (for definition, see glossary) for a. the main objective of the sampling and testing programme is to check the compliance of products on the market. Web. Finished Product Sampling Guidelines.
From digitalsampling.wordpress.com
Digital Product Sampling 101 Digital Product Sampling Finished Product Sampling Guidelines in the marketing authorisation application, the applicant should determine the most appropriate means for reaching the stated. sampling comprises the operations designed to select a portion of a pharmaceutical product (for definition, see glossary) for a. 5.2.3 finished product sampling should be done online as required in the product specification, preferably. who prequalification of a finished. Finished Product Sampling Guidelines.
From www.famisourcing.com
AQL Sampling 101 Meaning, Tables, Levels for Inspection Finished Product Sampling Guidelines what are the sampling requirements for sterility testing when a finished product batch of a terminally sterilised medicinal. 5.2.3 finished product sampling should be done online as required in the product specification, preferably. the who expert committee on specifi cations for pharmaceutical products adopted in 1999 the guidelines entitled who good. who prequalification of a finished. Finished Product Sampling Guidelines.
From pharmablog.in
Sampling of Intermediate and Finished Product SOP PharmaBlog Finished Product Sampling Guidelines sampling comprises the operations designed to select a portion of a pharmaceutical product (for definition, see glossary) for a. what are the sampling requirements for sterility testing when a finished product batch of a terminally sterilised medicinal. the main objective of the sampling and testing programme is to check the compliance of products on the market. Web. Finished Product Sampling Guidelines.
From www.slideshare.net
SAMPLING METHODS Finished Product Sampling Guidelines the who expert committee on specifi cations for pharmaceutical products adopted in 1999 the guidelines entitled who good. what are the sampling requirements for sterility testing when a finished product batch of a terminally sterilised medicinal. the main objective of the sampling and testing programme is to check the compliance of products on the market. who. Finished Product Sampling Guidelines.
From pharmablog.in
Sampling, Testing and Approval/Rejection of Packaging Material SOP Finished Product Sampling Guidelines in the marketing authorisation application, the applicant should determine the most appropriate means for reaching the stated. sampling comprises the operations designed to select a portion of a pharmaceutical product (for definition, see glossary) for a. 5.2.3 finished product sampling should be done online as required in the product specification, preferably. who prequalification of a finished. Finished Product Sampling Guidelines.